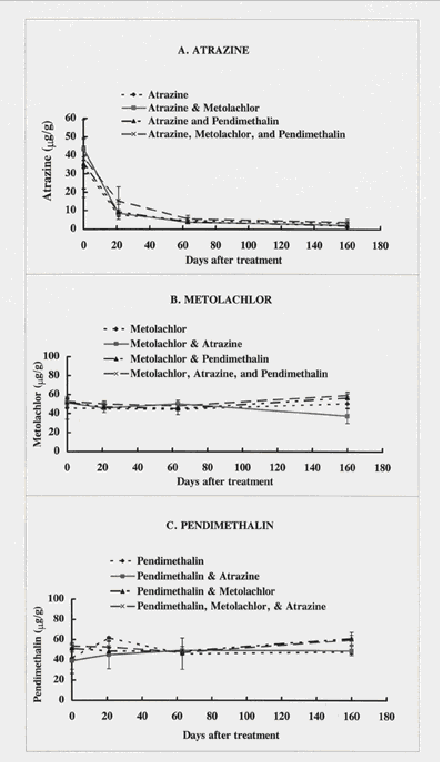
 Figure 1.
Concentrations of atrazine (1a), metolachlor (1b), and pendimethalin (1c) in the herbicide
interaction study
Figure 1.
Concentrations of atrazine (1a), metolachlor (1b), and pendimethalin (1c) in the herbicide
interaction study
Pesticide-Contaminated Soil Studies: Part I. Effects of Aging Herbicide Mixtures on Herbicide Degradation, Soil Respiration and Plant Survival. Part II. Phytoremediation Study with Native Prairie Grasses
J.C. Anhalt1, E.L. Arthur2, A. Chouhy2, T.A. Anderson3, and J.R. Coats2
1National Soil Tilth Laboratory, Department of Microbiology, Immunology, and Preventive Medicine (MIPM), Iowa State University, Ames, IA 50011, Phone: (515) 294-9602, FAX: (515) 294-8125, Email: janhalt@iastate.edu;2Pesticide Toxicology Laboratory, Department of Entomology, Iowa State University, Ames, IA 50011-3140, Phone: (515) 294-9823, FAX: (515) 294-9823; 3The Institute of Wildlife and Environmental Toxicology, Clemson University, Pendleton, SC 29670, Phone: (864) 646-2225, FAX: (864) 2277
Abstract
Studies were conducted on soils taken from a pesticide-contaminated site at an agrochemical dealership in Iowa. Herbicide interaction, soil respiration, plant survival, and germination studies were carried out. Three herbicides, atrazine, metolachlor, and pendimethalin, were applied individually and in all possible combinations to soil taken from a pesticide-contaminated site in Iowa. The rate of application for each chemical was 50 mg/g, representative of a moderate contamination level. At the end of each incubation period (0, 21, 63, and 160 d), soil respiration was measured by using an infrared gas analyzer; herbicide concentrations were determined by using a gas chromatograph; and germination and survival studies were conducted. Atrazine underwent considerable degradation in all soils, with no significant differences among soils treated with varying herbicide combinations. Metolachlor and pendimethalin underwent little or no degradation in all treatments.
A study conducted to determine the influence of native prairie grasses on the removal of aged pesticide contaminants was carried out in a field microplot study. A reduction in concentrations of extractable atrazine, metolachlor, and trifluralin from pesticide-contaminated soils was noted in soils vegetated with big blue stem, yellow indian grass, and switchgrass compared with nonvegetated soils.
Keywords: atrazine, metolachlor, pendimethalin, herbicide interaction, aged residues
Introduction
Pesticide-contaminated sites, such as those found at agrochemical dealership mixing and loading areas, are of great environmental concern. Soils at these sites are often contaminated as a result of accidental spillage with mixtures of pesticides at concentrations well above field-application rates. Phytoremediation has been a proposed approach for remediating pesticide-contaminated soils. Plants that are able to survive high concentrations of pesticide mixtures could contribute to pesticide waste degradation in soil as a result of intense microbial activity in the root zone or rhizosphere (Anderson, et al., 1994). The rhizosphere effect is important because of the potential reduction of pesticide wastes due to coincidental metabolism by microbial populations (cometabolism) or by catabolism of chemicals for use as a carbon or nitrogen source (Coats, 1993). Plants can also contribute to the removal of pesticide wastes through uptake into the plant tissue (Cunningham, et al., 1997). Little is known about the fate of pesticide mixtures in soil at point-source contamination levels. The current study was conducted to investigate whether interactions among herbicides in mixtures influence the degradation of individual herbicides. The effects of aging herbicide mixtures on soil respiration and plant germination and survival were also measured. Additionally, studies were conducted to determine the influence of native prairie grasses on the removal of pesticide wastes in soil.
Materials and Methods
Herbicide Interaction Study
A study was conducted to determine the effects of aging herbicide mixtures on soil respiration and germination and survival of sensitive plants using pesticide-contaminated soil from an agrochemical dealer site in Iowa.
Soil Sampling
Surface soils were sampled by removing the top 10 cm of soil with hand trowels. Soils were placed into zip-lock bags and transported on ice in a styrofoam cooler. Once in the laboratory, soils were stored at 4 oC until the study began. Soils from three independent composite samples were sieved and mixed well. Background concentrations of herbicides were determined in soils from the agrochemical dealership site by carrying out soil extractions and gas chromatographic analysis (Kruger, et al., 1997).
Soil Treatment
Soils were treated at 50 mg/g with seven treatments ( three replicates) (Table 1). After treatment, the solvent was evaporated by mixing the soil well, and subsamples were placed in jars to be incubated for either 0, 21, 63, or 160 d. The soil moisture was adjusted in each jar to a moisture content of -33 kPa. The jars were incubated in the dark at 25oC. At the end of each incubation period, soils were mixed well and divided into aliquots for soil respiration measurements, gas chromatographic analysis, and plant germination and survival tests.
Soil Respiration
Respiration rates of treated soils were measured at the end of each incubation period by using an infrared gas analyzer (LIRA Model 3000, Mine Safety Appliance Co.) (Edwards, 1982; Walton, et al., 1989). Ten-gram aliquots of soil were placed in 250 cm3 jars equipped with a stopper containing two glass tubes that were sealed with septa. The headspace of each jar was monitored for CO2 evolution at 24-h intervals for 7 to 10 d. Soil respiration of treated soils was compared to a non-pesticide-contaminated control soil for an indication of contaminant toxicity to soil microorganisms (Zelles, et al., 1986).
Germination Study
At the end of each incubation period, 20-g aliquots of soil from each treatment were planted with seeds from each plant species. The plant species used for this study were giant foxtail (Setaria faberi), birdsfoot trefoil (Lotus corniculatus), kochia (Kochia scoparia), canola (Brassica napus), and soybean (Glycine max). The date of germination and length of survival were recorded.
Chemical Analysis
At the end of each incubation length, twenty-gram aliquots were extracted three times with 40 ml ethyl acetate by shaking for 20 minutes. Soil extracts were concentrated by rotary evaporation and then brought to a volume of 10 mls. Samples were analyzed on a Shimadzu GC-9A gas chromatograph equipped with a flame-thermionic detector with the following conditions: column, glass packed with OV17, 1.8 m length; column temperature, 230 oC; injector temperature, 250 oC; detector temperature, 250 oC; carrier gas, helium; and flow rate, 35 mls/min.
Field Microplot Study
A field microplot study was conducted on soil collected from a pesticide-contaminated site in Iowa to determine if vegetation could enhance the removal of pesticide contaminants.
Soil Sampling
Pesticide-contaminated soils from an agrochemical dealership site in Iowa were sampled by removing the top 10 cm of surface soil with shovels. The soils were placed into 30-gallon drums, transported back to the laboratory, and stored outside at ambient temperature until their use.
Soil Treatment
Soils were spiked additionally with atrazine (100 mg/g), metolachlor (25 mg/g), and trifluralin (25 mg/g) by treating soils in a cement mixer. Subsamples of soil were taken from each cement mixer treatment (one per replication; n = 4). Aliquots of treated soils from each replication were transferred to one of three microplots which consisted of rectangular tubs measuring 15 cm deep, 24 cm wide, and 30 cm long. The floor of each tub was perforated to for allow drainage of water. Each tub containing soil was placed in a second, unperforated tub to trap contaminated water that would move through the treated soil. The microplots were placed outside in an Iowa State University research field and soils were allowed to age for 34 d before plants were added.
Prairie Grasses
During the aging period, native prairie grasses were planted in potting soil in small pots in the greenhouse, to be transplanted into the microplots. Qiu, et al. (1987) used prairie grasses for phytoremediation studies of polyaromatic hydrocarbons. The three species used in the current study were yellow indian grass (Sorghastrum natans), big bluestem (Andropogon gararidii), and switchgrass (Panicum virgatum). These species were chosen because of their ability to grow in the pretreated pesticide-contaminated soils used in this study. Big bluestem and switchgrass have been found to be tolerant to atrazine, while indian grass is susceptible in the seedling stage (Weimer, et al., 1988). Grasses were planted in the greenhouse in potting soil one week prior to transplanting them into the treated soils, with the hope of increasing the chance of survival of the grasses in the treated soils. Three soil treatments were included in this study: (1) single species-big bluestem; (2) mixed species-big bluestem, yellow indian grass, and switchgrass; and (3) no vegetation. Since rhizosphere microbial populations can differ qualitatively among plant species (Anderson, et al., 1993), it was hypothesized that a mixture of plants might have a greater effect on enhancing degradation of pesticide contaminants than would the single species. Miller, et al. (1989) noted differences in microbial make-ups of the rhizospheres of maize, wheat, and grass cultivars.
Thirty-four days post-treatment, microplots were either transplanted with a single species, a mixture of three species, or left unvegetated. At the time of planting, subsamples of soil were taken from the microplots to determine the concentrations of herbicides remaining. Nineteen days after planting, microplots were once again sampled and analyzed for herbicide concentrations.
Chemical Analysis
Twenty-five grams (dry weight) of soil from each microplot were extracted three times with 50 ml ethyl acetate by shaking for 20 minutes. Soil extracts were concentrated by rotary evaporation and then brought to a volume of 10 mls. Samples were analyzed on a Shimadzu GC-9A gas chromatograph with conditions identical to those used in the herbicide interaction study.
Results and Discussion
Herbicide Interaction Study
Herbicide Degradation
Background concentrations for atrazine, metolachlor, and pendimethalin are shown in Table 2. The background concentration of atrazine was 0.6 mg/g. Background concentrations of pendimethalin and metolachlor were 9 and 6 mg/g in soil, respectively.
Of the three herbicides applied, atrazine underwent the greatest amount of degradation, decreasing from 50 mg/g on day zero to less than 11 mg/g remaining in all treatments after 21 d of incubation. After 160 days, less than 3 mg/g of atrazine was extractable from soil (Figure 1a). The degradation of individual chemicals was not affected by the presence of one or more of the other chemicals. Dzantor and Felsot (1991) found that alachlor degradation was similar in soils treated individually or in combination with atrazine, metolachlor, and trifluralin for soils treated at 10 mg/kg. They also reported that minimal degradation occurred in soils treated with excessive amounts of these herbicides (10,000 mg/kg) after one year of incubation. Rapid degradation of atrazine in soil from this agrochemical dealer site prompted another study in our laboratory. This study looked at atrazine degradation in soils from other sites to determine if this phenomenon was widespread in soils that have had long-term exposure to atrazine spills. Of the eight soils tested, less than half exhibited rapid mineralization of atrazine in soil applied at 50 mg/g (Kruger, et al., 1997). Enhanced mineralization of atrazine was noted in rhizosphere soils from a pesticide-contaminated site compared with nonvegetated soils (Perkovich, et al., 1996).
There were no significant differences in concentrations of metolachlor from the day of treatment to 160 days post treatment, with a mean concentration of metolachlor after 160 days ranging from 43 to 59 mg/g. Background concentrations of metolachlor in the soil contributed to concentrations exceeding the treatment level at the beginning of this study (50 mg/g). There were no differences among soils treated with any of the combinations of herbicides (Figure 1b).
Pendimethalin concentrations did not differ statistically from the day of treatment to 160 days of incubation (Figure 1c). Concentrations of pendimethalin extractable from soils at the end of this study ranged from 48 to 61 mg/g.
Although considerable mixing of soils was done prior to taking subsamples for gas chromatographic analysis, variable concentrations were still seen among the replications. While using soils from a pesticide-contaminated site is important in understanding the fate of chemicals under these conditions, the heterogeneity of background contamination complicates statistical analysis for treatment effects.
Soil Respiration
For the day 0 soils, soil respiration for all treatments was depressed for the first seven days after herbicide treatment and then began to increase steadily, with the highest reading on day 10 in soils treated with atrazine alone. Soil respiration was low for day 21, 63, and 160 soils.
Germination Study
Of the plants tested in this study, only two plant species, soybean and canola, were able to germinate in soils of all chemical treatments aged for 0, 21, 63, or 160 days. Generally, the trend noted for these two species was a decrease in the length of time required for plant germination to occur with increased aging of herbicide residues. Immediately following chemical treatment, germination time for soybean and canola ranged from 5 to 12 and 4 to 6 days, respectively. Twenty-one days post-treatment, canola germination time in soils receiving individual chemical treatments was reduced to 2 days, and ranged from 4 to 6 days for mixtures of herbicides. After herbicides had aged in soil for either 63 or 160 days, the germination time for canola and soybean was reduced to 2 days for all chemical treatments. The germination of birdsfoot trefoil was inhibited in most treatments until after the 160-day aging period, with a very short survival of 1 to 6 days. No patterns were seen for kochia or giant foxtail, for which germination was sporadic even in control soils. For future studies, plant species which are easy to germinate and grow under normal conditions should be used for ease of data interpretation.
Decreased bioavailability or degradation of herbicide residues in the soil over time likely have contributed to the decreased germination times noted in this study for canola and soybean. Further studies assessing the bioavailability to other sensitive plants, as well as to terrestrial and aquatic organisms would provide more insight into the changes in bioavailability of pesticides during their aging process in soils.
Field Microplot Study
Several herbicide contaminants were detected in background samples analyzed by gas chromatography prior to the study. Atrazine concentrations were less than 1 mg/g in all but one sample. In one sample, the concentration of atrazine was 48 mg/g, demonstrating the heterogeneity of contamination at the agrochemical dealer site. Metolachlor concentrations were as high as 11 mg/g. Two dinitroaniline herbicides were detected in soils from the site. Trifluralin was detected at trace levels (less than 0.1 mg/g), while pendimethalin concentrations ranged from 105 to 112 mg/g.
Preliminary results indicate that vegetation of contaminated soils with prairie grasses decreased the amounts of pesticide contaminants in soils. For metolachlor, concentrations were significantly less in soils vegetated with a mixture of big blue stem, yellow indian grass, and switchgrass, compared with nonvegetated soils (ANOVA; p = 0.0004). Concentrations of metolachlor in the mixed-species and nonvegetated microplots were 10 mg/g and 24 mg/g, respectively, 19 d post-planting (Figure 2). In the soils vegetated with the mixed species, this is less than half the concentration initially applied to the microplots (25 mg/g).
Concentrations of trifluralin were significantly less in soil vegetated with the mixed species of prairie grasses compared with nonvegetated soils (ANOVA; p = 0.0001), with 15 mg/g and 20 mg/g extractable from soils 19 days post planting. There were no significant differences between soil vegetated with the single species, big blue stem, compared with either of the other two treatments (nonvegetated or mixed species) (Figure 3). In a soil incubation study investigating the degradation of atrazine and trifluralin in soils from an agrochemical dealer site, significantly less of each herbicide was extractable from rhizosphere soil than from nonvegetated soils (Anderson, et al., 1994).
Atrazine concentrations were significantly less in soils vegetated with single or mixed species (11 mg/g) compared with nonvegetated soils (13 mg/g) 19 d after plants were added (ANOVA; p = 0.03). Kruger, et. al. (1997) noted that Kochia scoparia had a positive effect on the degradation of atrazine in soil, with less extractable atrazine in soils vegetated with Kochia plants compared with nonvegetated soils.
Pendimethalin concentrations, from contamination at the site, were less in all treatments 19 days after vegetation was added to the study (~ 75 mg/g). There were no differences among vegetated and nonvegetated soils. Pendimethalin is intensely difficult to desorb from soil, making leaching a very small concern. It has also been noted that pendimethalin will stay in the soil even if water is applied to the field at 100% field capacity daily (Cooper, et al., 1994) (Berayon and Mercado, 1983).
Conclusions
1. Atrazine underwent rapid degradation in all soil treatments of the herbicide interaction study, with a 50% reduction by the end of 21 days.
2. Metolachlor and pendimethalin underwent little or no degradation in all soil treatments of the herbicide interaction study.
3. As the aging time of herbicide residues increased, germination time of canola and soybean decreased.
4. Atrazine concentrations were significantly less in plots vegetated with mixed and single prairie grass species compared with nonvegetated plots.
5. Metolachlor and trifluralin concentrations were significantly less in field microplots vegetated with the combination of three prairie grass species compared with nonvegetated plots.
6. The results of this field microplot study indicate that prairie grass species look promising for phytoremediation of pesticide-contaminated soils.
Acknowledgments
This work was partially funded by Novartis Crop Protection (Greensboro, NC), the Great Plains/Rocky Mountain Hazardous Substance Research Center, and the U. S. Environmental Protection Agency (USEPA) (Cooperative Agreement CR-823864-01). Partial support for E. L. A. was provided by a USEPA Graduate Fellowship. Journal Paper No. J-_______ of the Iowa Agriculture and Home Economics Experiment Station, Ames, IA. Project No. 3187.
References
Anderson, T. A., and J. R. Coats. 1995. Screening rhizosphere soil samples for the ability to mineralize elevated concentrations of atrazine and metolachlor. J. Environ. Sci. Health-Part B30:473-484.
Anderson, T. A., E. L. Kruger, and J. R. Coats. 1994. Enhanced degradation of a mixture of three herbicides in the rhizosphere of herbicide-tolerant plants. Chemosphere 28:1551-1557.
Anderson, T. A., E. A. Guthrie, and B. T. Walton. 1993. Bioremediation in the rhizosphere. Environ. Sci. Technol. 27:2630-2636.
Anderson, T. A., Kruger E. L., and J. R. Coats. 1994. Enhanced degradation of a mixture of three herbicides in the rhizosphere of a herbicide-tolerant plant. Chemosphere 28:1551-1557.
Arthur E. L., T. A. Anderson, and J. R. Coats. 1997. Evaluation of the degradative capabilities of soils from pesticide-contaminated sites: Influence of two plant species on the degradation of aged residues. Environmental Toxicology and Chemistry (in preparation).
Berayon, B. F. and B. L. Mercado. 1983. Persistence of pendimethalin in the soil. Phil. Agr. 66:367:378.
Coats, J. R. 1993. What happens to degradable pesticides. Chemtech 23:25-29.
Cooper, J. F., S. Q. Zheng, L. Palcy, and C. M. Coste. 1994. Behaviour of pendimethalin in tropical and mediterranean plain field condition. J. Environ. Sci. Health, B29(3):443-457.
Cunningham, S. D., J. R. Shann, D. E. Crowley, and T. A. Anderson. 1997. Phytoremediation of contaminated water and soil. In E. L. Kruger, T. A. Anderson, and J. R. Coats (eds.). Phytoremediation of Soil and Water Contaminants, American Chemical Society Symposium Series 664, Washington, D. C., pp 12-17.
Dzantor, E. K., and A. S. Felsot. 1991. Microbial responses to large concentrations of herbicides in soil. Environ. Toxicol. Chem. 10:649-655.
Edwards, N. T. 1982. A timesaving technique for measuring respiration rates in incubated soil samples. Soil Sci. Am. J. 46:1114-1116.
Kruger, E. L, J. C. Anhalt, D. Sorenson, B. Nelson, A. L. Chouhy, T. A. Anderson, and J. R. Coats. 1997. Atrazine degradation in pesticide-contaminated soils: Phytoremediation potential. In E. L. Kruger, T. A. Anderson, and J. R. Coats (eds.) Phytoremediation of Soil and Water Contaminants, American Chemical Society Symposium Series 664, Washington DC, pp 54-64.
Miller, H. J., G. Henken, and J. A. van Veen. 1989. Variation and composition of bacterial populations in the rhizospheres of maize, wheat, and grass cultivars. Can. J. Microbiol. 35:656-660.
Perkovich, B. S., T. A. Anderson, E. L. Kruger, and J. R. Coats. 1996. Enhanced mineralization of 14C-atrazine in Kochia scoparia rhizospheric soil from a pesticide-contaminated site. Pesticide Science. 46:391-396.
Qiu, W., T. W. Leland, S. I. Shah, D. L. Sorenson, and E. W. Kendall. 1997. Field study: Grass remediation for clay contaminated with polycyclic aromatic hydrocarbons. In E. L. Kruger, T. A. Anderson, and J. R. Coats (eds.) Phytoremediation of Soil and Water Contaminants, American Chemical Society Symposium Series 664, Washington, D. C., pp. 186-189.
Walton, B. T., T. A. Anderson, and M. S. Hendricks. 1989. Physicochemical properties as predictors of organic chemical effects on soil microbial respiration. Environ. Toxicol. Chem. 8:53-63.
Weimer, M. R., B. A. Swisher, and K. P. Vogel. 1988. Metabolism as a basis for differential atrazine tolerance in warm-season forage grasses. Weed Sci. 36:436-440.
Zelles, L., L. Scheunert, and F. Korte. 1986. Comparison of methods to test chemicals for side effects on soil microorganisms. Ecotox. Environ. Safety 12:53-69.
Table 1. Seven treatments for herbicide interaction study.
| Treatment ID | Herbicides |
| A50 | atrazine |
| M50 | metolachlor |
| P50 | pendimethalin |
| A50M50 | atrazine & metolachlor |
| M50P50 | metolachlor & pendimethalin |
| A50M50P50 | atrazine, metolachlor, & pendimethalin |
Table 2. Background concentrations of herbicide contaminants in
soil from an agrochemical dealership of Iowa used for the herbicide
interaction study.
| Herbicide | Concentration
(µg/g) |
Standard Deviation
of the mean |
| Atrazine | 0.6 | 0.03 |
| Metolachlor | 6.1 | 0.9 |
| Pendimethalin | 9.2 | 0.2 |
 Figure 1.
Concentrations of atrazine (1a), metolachlor (1b), and pendimethalin (1c) in the herbicide
interaction study
Figure 1.
Concentrations of atrazine (1a), metolachlor (1b), and pendimethalin (1c) in the herbicide
interaction study
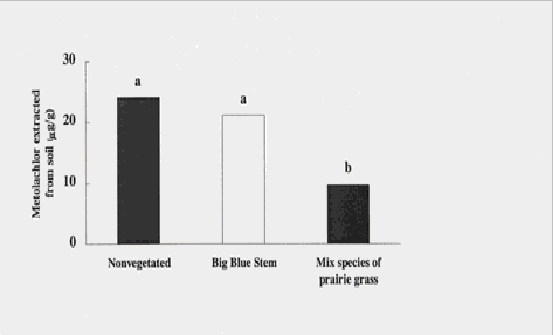 Figure 2. In soils planted with a mixture of prairie grasses, Metolachlor was significantly
less than in soils planted with one species or left unvegetated. Means with the same letter
are not significantly different (ANOVA).
Figure 2. In soils planted with a mixture of prairie grasses, Metolachlor was significantly
less than in soils planted with one species or left unvegetated. Means with the same letter
are not significantly different (ANOVA).
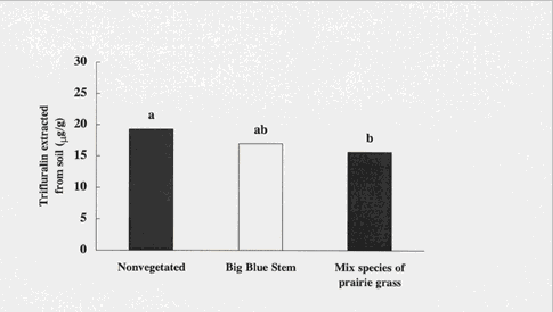 Figure 3. Trifluralin concentrations were significantly less in soils vegetated with a
mixture of prairie grasses compared to soils left unvegetated. Means with the same letter
are not significantly different (ANOVA).
Figure 3. Trifluralin concentrations were significantly less in soils vegetated with a
mixture of prairie grasses compared to soils left unvegetated. Means with the same letter
are not significantly different (ANOVA).
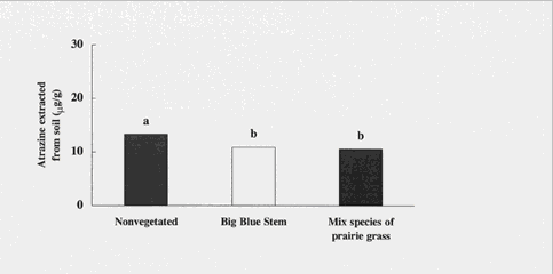 Figure 4. Atrazine concentrations were significantly less in soils vegetated with a mixture
of prairie grasses compared to nonvegetated soils. Means with the same letter are not
significantly different (ANOVA).
Figure 4. Atrazine concentrations were significantly less in soils vegetated with a mixture
of prairie grasses compared to nonvegetated soils. Means with the same letter are not
significantly different (ANOVA).
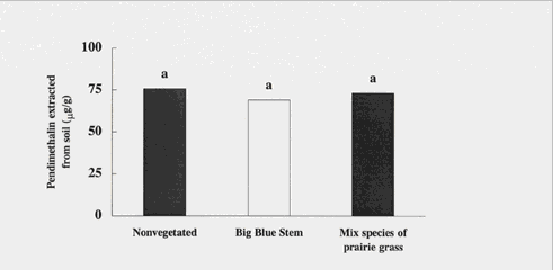 Figure 5. Concentrations of pendimethalin were reduced in soils as compared to original
concentrations of greater than 110 µg/g, and concentrations were similar in treatments 19
d post-planting. Means with the same letter are not significantly different (ANOVA).
Figure 5. Concentrations of pendimethalin were reduced in soils as compared to original
concentrations of greater than 110 µg/g, and concentrations were similar in treatments 19
d post-planting. Means with the same letter are not significantly different (ANOVA).