Phytoremediation of Trichloroethylene and Carbon Tetrachloride: Results From Bench to Field
L.A. Newman1, C. Bod2, N. Choel, R. Crampton3, R. Cortellucci4, D. Domroes4, S. Doty1, J. Duffy4, G. Ekuan2, D. Fogel2, R. Hashmonay3, P. Heilman2, D. Martin1, I.A. Muiznieks1, T. Newman5, M. Ruszaj4, T. Shang1, B. Shurtleffl, S. Stanleyl, S.E. Strand6, X. Wang6, J. Wilmouth1, M. Yost3 and M.P. Gordon1
1Department of Biochemistry, Box 357350, University of Washington, Seattle, WA 98195, Phone: (206) 543-5504; 2Washington State University, Puyallup Research and Extension Center, Puyallup, WA, Phone: (206) 840-4522; 3Department of Environmental Health, University of Washington, Seattle, WA 98195, Phone: (206) 685-7243; 40ccidental Chemical, 2801 Long Road, Grand Island, NY 14072, Phone: (716) 773-8476; 50ccidental Chemical, 605 E Alexander Avenue, Tacoma, WA 98421, Phone: (206) 383-2661; 6College of Forest Resources, University of Washington, Seattle, WA 98195, Phone: (206) 543-5350
Abstract
Remediation of contaminated sites using plants, or phytoremediation, is one of the most promising new technologies for remediation. As with any new technology, solid data concerning the efficacy of this method needs to be produced before commercial groups are willing to implement the technology. This work shows that axenic poplar cell cultures produced from hybrid poplar H-11-11 (Populous trichocarpa x P. deltoides) are capable of independently oxidizing trichloroethylene (TCE) to expected metabolites. It also demonstrates that young rooted cuttings, when placed in metabolic chambers or grown under greenhouse conditions, are capable of taking up and transpiring TCE. Further tests include a pilot-scale remediation project simulating what would be seen on a contaminated site. After one year of exposure to TCE, the data shows that hybrid poplars were able to extract significant amounts of TCE from the water stream. Additionally, at dose concentrations up to 50ppm, there is no apparent effect on the above ground growth of the trees. Continued use of bench and pilot-scale facilities will allow the testing of different species of plants challenged with a wide range of chemicals.
Keywords: phytoremediation, poplar, trichloroethylene, carbon tetrachloride
Introduction
Trichloroethylene (TCE) is one of the most widespread contaminants in the environment of the United States (Rajagopal, 1986). TCE can be stable in groundwater and can persist for decades in DNAPLS. The difficulty in pinpointing the location of these pools of TCE can greatly hinder attempts at remediation. The fact that TCE is a suspected carcinogen (National Cancer Institute, 1976) has lent urgency to cleanup efforts. The principle methods for remediating groundwater contaminated with TCE are pumping water from the aquifer and stripping the TCE by aeration or by charcoal absorption. These procedures can take years or decades and can be very expensive (Travis and Doty, 1990). Other techniques utilize bacteria to degrade TCE, although this procedure often requires toxic inducers such as toluene or phenol for degradation to occur (Hopkins, et al., 1993).
Recent research investigating the use of plants to remediate environmental pollution is yielding exciting results. With some species of plants the major site of TCE degradation appears to be in the rhizosphere and the actual role of the plant in remediation may be to supply nutrients to the rhizosphere organisms (Anderson and Walton, 1995; Anderson, Guthrie and Walton, 1993).
We have investigated three clones of Populus, designated H11-11, as a means to remediate TCE pollution. Poplar was chosen for a variety of reasons. Poplar has a very wide geographical distribution and can be grown from southern Alaska into Central America. Members of the species can be easily crossed sexually. Propagation by cuttings is simple yielding clones of a given individual. Poplars can be grown axenically in culture and exogenous genes can be incorporated into the poplar genome. Thus there is the potential of incorporating genes that will more completely mineralize TCE or other pollutants. The College of Forest Resources at the University of Washington has worked with poplars for over twenty years, and has accumulated an enormous amount of background concerning the physiology and genetics of this genus. The absorption surface of roots in a stand of poplars is enormous and can approach 300,000 km/ha. The water usage under the warm, arid conditions of eastern Washington State is about 140 cm per year in a stand of 5-year-old trees at a density of 1,750 trees/ha.
The investigations discussed in this chapter first utilized axenic cultures of a poplus trichocarpa x deltoides H11-11 tumor cells, then small plants treated with water containing TCE, and, finally, a number of experimental cells which mimicked field trials. We also developed a bioreactor that enabled us to account for the distribution of most of the TCE metabolized by small H11-11 plants .
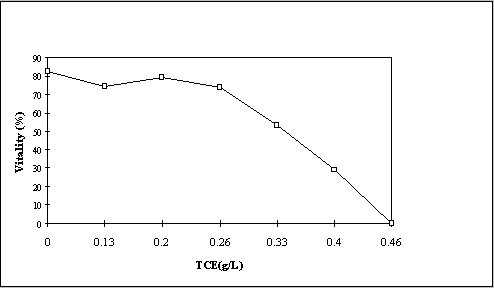
Axenic tumor cell experiments
The axenic H11-11 tumor cells were produced by transforming shoots of H11-11 with Agrobacterium tumefaciens A281. Tumor cells are used because they can be easily grown on simple minimal medium (Murashige and Skoog basal salt medium) (Murashige, 1962). The cells were grown with shaking and illumination in the above medium at various degrees of saturation of TCE for three or five days at 22oC. The toxicity of TCE towards the H-11-11 cells was determined by the vital stain, trypan blue (Figure 1). In order to study the metabolites of TCE, the poplar cells were incubated with 0.080 gm TCE per Liter. The suspension was then centrifuged, and a sample of the cells was extracted at 20oC with lN H2SO4/10% NaCl, then three times with methanol, and then 3 times with methyl tertiary butyl ether. In order to test for the presence of di- and trichloroacetic acid, a second batch of cells was extracted with sodium hydroxide, the extracts acidified, and back extracted with methyl tertiary butyl ether, and the organic acids in the ether extract esterified with diazomethane. The methyl esters were then analyzed. The results of these analyses are given in the accompanying table (Table 1).
The cell cultures were also tested for the formation of l4C-CO2 from TCE. The gases evolved during four- or ten-day incubations with 14C-TCEx, were trapped in NaOH and confirmed to be CO2. The cells and media were separated by centrifugation and the amount of 14C remaining in the media determined. The insoluble extracted cell residue was combusted and the resulting CO2 analyzed for radioactivity. The products from the TCE metabolism, trichloroethanol, di- and trichloroacetic acid, and CO2, are also among the products produced by the activity of rat and mouse hepatic cytochrome P450 (Dekant, 1986). These findings suggest that the oxidative metabolism of TCE in poplars is similar to the processes in mammals.("green liver" hypothesis.)
Whole plant experiments
Whole plants were rooted in PVC pipes, 20.5 cm diameter, containing a 30cm bottom layer of sand with 60cm of soil overlay. The plants were dosed on water alone, or with 50 ppm TCE by addition directly to the sand layer through an inner watering tube. A total of 8gm of TCE was added to each plant over a period of eight months. During the period of growth, transpiration of TCE was assayed by enclosing the leaves in plastic bags and pulling the air through a charcoal filter for 0.5 hours. The leaves transpired approximately 1.0µg of TCE/leaf/hour. The results were highly variable and indicated transpiration values from undetectable to about 1.6 micrograms TCE per leaf/hour. After eight months, the plants in PVC pipes were harvested and various morphological measurements were taken (Table 2). The major difference noted was that the density of the fine roots in the sand layer of TCE exposed plants was less than that of the controls. The plant tissues were analyzed for metabolites of TCE (Table 3). The nature and levels of the chlorinated metabolites suggest that the TCE is oxidized as it moves from bottom roots to the upper sections of the crown of the plant, especially the leaves. These results, together with the products derived from the axenically-cultured poplar cells, strongly argue for a role of the plant in the metabolism of TCE, in addition to the well known degradation of TCE by soil microorganisms.
Mass balance studies
The mass balance of TCE in hybrid poplar trees was determined in a bioreactor. The crown, stem, and roots of the plant had to be kept separate so that the transpiration of the compound by each organ could be determined without interference by volatiles from other plant tissues or the soil. The major problems we faced are due to the volatility of TCE and its suspected metabolites, and the absorption of these materials by commonly used laboratory materials such as rubber, tygon, and various sealants. The chamber is shown in the accompanying diagram (Figure 2) and is constructed of glass, aluminum foil, and inert inorganic materials. Note that the separate chambers for roots, stems, and crown are independently aspirated to prevent volatile transpirates from leaking into other chambers. The TCE is added to the bottom chamber to simulate contaminated soil; controls show only 0.03% leakage of the TCE into the crown chamber.
Small rooted poplar cuttings (ca. 20 cm tall) were planted in a peat moss/vermiculite mixture in the root chamber and 14C-TCE added to the roots at 5 ppm. After seven days, approximately 0.8% of the added 14C was detected in the transpirate and trace amounts were converted to CO2.
Several conclusions can be drawn from these three laboratory experiments. First, that TCE can be taken up by poplars. Second, some of the material is transpired by the plants and some is metabolized to known extractable metabolites. Finally, some non-extractable material is fixed in tissue; the nature of incorporation in tissues is under investigation. We predict that the contribution of each of these pathways towards the removal of TCE would depend upon variables such as concentration of TCE, size of tree, type of soil, temperature, light intensity, relative humidity, wind velocity, etc.
Controlled field trials
A controlled field trial was set up in collaboration with the Occidental Chemical Corporation in which an artificial aquifer was constructed. Double walled cells, 3.7 m x 6.1 m x 1.5 meters deep, were constructed of 60-gauge high density polyethylene plastic sheeting. The bottoms had about 0.3 m of sand overlaid with 1.1 m of Sultan silt loam. In order to ensure a uniform flow of input water, a "T"-shaped input pipe was utilized at the bottom and a 1/40 slope was oriented toward the effluent well. Four cells were planted with fifteen 30 cm plants of Populus trichocarpa x P deltoides, H11-11. Two of these cells were dosed with water containing 50 ppm TCE while the other two cells received only water. The fifth cell, which had no vegetation, also received water with 50 ppm TCE. Each of the cells received the same volume of liquid; however, variable amounts of liquid were pumped out of the cells to maintain a level of about 20 cm of liquid in the bottom of the cells.
About nine weeks after injection of TCE into the cells, breakthrough of TCE and related metabolites, particularly cis-1,2-dichloroethylene, occurred in the cells which did not contain trees. The occurrence of this compound is indicative of anaerobic bacterial dehalogenation of TCE. In cells with trees, very little TCE or metabolites could be detected in the effluent until after leaf drop (Figure 3). The stems, roots, and leaves of the trees exposed to TCE showed the above products of oxidative metabolism, which were also seen in the greenhouse studies and tissue culture experiments. The one-year-old trees (ca. 3.5 meters tall) were very effective in removing TCE from the input water. These experiments are being continued.
Conclusion
The results of the above experiments show that TCE has multiple fates in poplars. The trees transpire unaltered TCE. The compound also can undergo oxidation to chloromethyl derivatives, trichloroethanol, dichloroacetic acid, trichloroacetic acid, or complete mineralization to CO2, which are oxidative metabolites found in mammalian livers. Variable amounts of TCE are found fixed in insoluble, nonextractable residues. Plant uptake coupled with TCE metabolism serves to remove TCE from groundwater and from the soil environment. The details of these reactions and methods for increasing the mass flow through these pathways are subjects of current investigations. We are also attempting to introduce enzymes into poplars to increase their tolerance to higher levels of TCE and to increase the fraction of TCE that is mineralized to CO2.
These experiments show that actively metabolizing poplars are able to intercept a moving plume of TCE-contaminated water and reduce the levels of this compound significantly. The system shows promise where there is sufficient space to plant trees and when the roots can reach the contaminated region.
Acknowledgments
This work was generously supported by grants from Occidental Chemical Corporation, the U. S. Environmental Protection Agency 10#R822329-01-3, and the U. S. National Institute of Environmental Health and Safety Superfund Grant # 2P42 ES04696-09.
References
Defalque, R. J. Clin. Pharmacol. Ther., 1961, 2, 665-688.
Abrahamson, K.; Ekdahl, A.; Collen, J.; Pedersen, M. Limnol Oceanographic, 1995, 40, 1321-1326.
Rajagopal, R. Environ. Prof:, 1986, 8, 244-264.
National Cancer Institute. Carcinogenesis bioassay of trichloroethylene., 1976, CAS no. 76-01 -6. U. S. Department of Health, Education and Welfare publication (NIH) 76-802.
Travis, C. C.; Doty, C. B. Environ. Sci. and Technol., 1990, 24, 1464-1466.
Hopkins, D.; Munakan, J.; Semprini, L.; McCarty, P. L. Environ. Sci Technol., 1993, 27, 2542-2547.
Anderson, T. A.; Walton, B. T.Environ. Toxicol. and Chem. 1995, 14:12, 2041-2047.
Anderson, T. A.; Guthrie, E. A.; Walton, B. T. Environ. Sci. and Technol. 1993, 27:13, 2630-2636.
Murashige, T.; Skoog. F. Physiol. Plant., 1962, 15, 473-497.
Dekant, W. New Concepts and Developments in Toxicology.; Elsevier Science Publishers
B. V. Biomedical Division.: Amsterdam, The Netherlands, 1986, P. L. Chambes,
P. Gehring and F. Sakai, eds. Metabolic conversion of tri- and tetrachloroethylene:
formation and deactivation of genotoxic intermediates, pp. 211-221.
Table 1. Amounts of TCE and metabolites found in supernatant and axenic cells exposed to TCE. (Amounts given are nanograms of TCE or metabolite per gram of sample.
ND = not detected at stated limit.)
| TCE | Chloral
Hydrate |
Trichloro-
ethanol |
Dichloro-
acetic acid |
Trichloro-acetic acid | |
| Control 1
pellet supernatant |
ND40 ND40 |
ND40 ND40 |
ND40 ND40 |
ND10 ND10 |
ND10 ND10 |
| Control 2
pellet supernatant |
ND40 ND40 |
ND40 ND40 |
ND40 ND40 |
ND10 ND10 |
ND10 ND10 |
| Exposed - batch 1
pellet supernatant |
ND40 2000 |
ND40 ND40 |
60 760 |
12000 1600 |
ND10 ND10 |
| Exposed - batch 2
pellet supernatant |
ND40 ND40 |
ND40 ND40 |
80 110 |
39000 3800 |
130 30 |
Table 2. Comparison of TCE-treated and control plants.
(Measurements of plants exposed to TCE were compared
to control plants grown under the same greenhouse conditions
for 8 months.)
| Plant Parameter | % of control |
| Height | 80-92 |
| Stem weight | 70-72 |
| Leaf area | 75-78 |
| Number of leaves | 50-80 |
| Root weight | 52-70 |
| Length of fine roots | 22-32 |
Table 3. Amounts of TCE and metabolites found in tissues of plants exposed to TCE under greenhouse conditions. (Amounts given are nanogram of TCE or metabolite per gram of sample. ND = not seen at stated detection limit.)
| tissue | clone* | TCE | Chloral
hydtrate |
Trichlo-
ethanol |
Dichloro
acetic acid |
Trichloro
acetic acid | |
| control 1 | leaves
stems |
A | ND40
ND40 |
ND40
ND40 |
ND40
ND40 |
ND10
ND10 |
ND10
ND10 |
| control 2 | leaves
stems |
B | ND40
ND40 |
ND40
ND40 |
ND40
ND40 |
ND10
ND10 |
25
ND10 |
| control 3 | leaves
stems |
B | ND40
15 |
ND40
ND40 |
ND40
ND40 |
ND10
ND10 |
ND10
ND10 |
| TCE 1 | leaves
stems |
A | 13
770 |
ND40
ND40 |
180
140 |
ND10
ND10 |
1100
31 |
| TCE 2 | leaves
stems |
B | 49
1900 |
ND40
ND40 |
19
170 |
180
ND10 |
7200
22 |
| TCE 3 | leaves
stems |
B | 27
1300 |
ND40
ND40 |
24
125 |
ND10
ND10 |
2100
100 |
| TCE 4
Roots: |
upper middle lower |
A |
13 150 640 |
ND40 ND40 ND40 |
200 110 31 |
320 25 270 |
44 21 44 |

Figure 1. Toxicity of TCE on H11-11 tumor cells. Viability was determined by trypan
blue exclusion as only dead cells are stained by this dye. One hundred cells per point are
counted. The error per point is about ±2%.
Figure 2. Diagram of a bioreactor. The temperature of the carbon tubes was maintained
at 40o C by heat tape to prevent condensation of water.
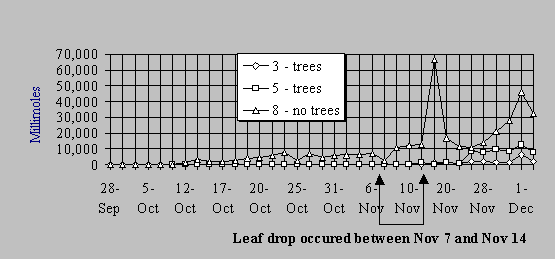
Figure 3. Millimoles of TCE and metabolites recovered in effluent water 28 September through 4 December 1995. Cells 3 and 5 contain trees. Cell 8 is the non-vegetated control. The spikes on November 17 and December 1 are due to flooding during periods of very heavy rainfall.