Detection of Divalent Transition Metal IONs in Complex Media BY Capillary ELECTROPHORESIS
J.D. Sgammato, A. DiIorio, and T.C. Crusberg
Worcester Polytechnic Institute, Department of Biology and Biotechnology, Worcester, MA, 01609
Abstract
A method was developed for the analysis of divalent metal ions in complex media. Research into the bioremediation of metals requires exploratory methods for analyzing those metals in growth media. Capillary electrophoresis uses small (<500
mL) samples, permitting multiple analyses over time without introducing volumetric effects. But growth media often contain concentrations of other cations high enough to interfere with resolution of the metals in question.A capillary electrophoretic method was developed that analyzes Cu, Ni, and Zn at concentrations as low as 10 mg/L in nutrient media containing 540 mg/L Na, 270 mg/L K, 50 mg/L Mg, and 27 mg/L Ca. The analysis uses 500
mL samples, permitting aliquots to be taken during the course of a shake-flask experiment without introducing volumetric errors. Multiple analyses can be made from the same sample. A sample takes 10 minutes to run, with a 2.5 minute purge and 5-second injection between samples, and is automated, permitting overnight analysis. No sample preparation was required.This method is useful for determining rates of metal uptake, optimizing nutritional requirements, for microbial growth rates, and the effect of varying environmental factors for metal-sorbing organisms. This method has been applied to research on the copper-immobilizing fungus Penicillium ochro-chloron.
Keywords: capillary electrophoresis, transition metals, complex media, capillary ion analysis, copper
Introduction
Analysis of transition metal ions in simple aqueous solutions by capillary electrophoresis is well-established. Spectrophotometric and chemical analysis of the same ions in complex media is also well-established, especially those methods outlined in Standard Methods. Capillary electrophoresis methods have a number of advantages over many of the other methods: ability to quantitate multiple ions in a single sample, sample size, speed, and ease of use. Capillary electrophoresis is especially well-suited for analyzing samples generated in screening studies, due to its facility with multiple, small samples. But a capillary electrophoresis method suitable for transition metals in water was unsuitable for use in complex media.
Unlike water tested for compliance with federal discharge limits, the aqueous media used to produce microorganisms for bioremediation research has relatively high concentrations of the "physiological metals" (K+, Mg+, Na++, and Ca++), and the concentrations of these cations can vary widely between media. In bioremediation studies on the abilities of microorganisms to precipitate, absorb, or otherwise immobilize transition metals, high concentrations of these media components can interfere with the smaller peaks created by the metals being studied. A method was developed for the analysis of transition metal cations in microbial growth and maintenance media.
The experiments for which this method became the detection method involved exposing a population of the filamentous fungus Penicillium ochro-chloron to different concentrations of metals. The fungus precipitated the metal on and in the mycelia (Crusberg, et al., 1991). This method was developed to do rapid screening studies of the metal content of the media over time without introducing volumetric effects. It involves no sample preparation; 500-mL samples are withdrawn from the reactor into a sample vial and run. The time from taking the sample until seeing results is under 10 minutes. In this way we were able to reliably and conveniently track metal uptake versus time in different reactor configurations.
Materials
The salts used in preparation of media and standards were from SIGMA. The water was 18-megohm water from the laboratory water purification system.
The capillary electrophoresis instrument was a Waters Quanta 4000 with the Capillary Ion Analyzer v3.1 firmware card. Data was converted with a Waters SAT/IN box before being sent to a 486DX50 computer running Windows 3.1.1 and Waters Millennium Chromatography Manager software with the Capillary Ion Analysis option added. The capillary was from a reel of 75-mm I.D. polyimide-coated fused-silica capillary from Polymicro Technologies (Phoenix, AZ), cut to 60cm and prepared according to the instructions in the Quanta 4000 Operatorís Manual. Unsiliconized 500-mL sample vials (Fisher Scientific. Pittsburgh, PA) were used for all samples.
Methods
Capillary electrophoresis has been well-described by many other researchers and in several comprehensive texts.
Two different media were used- GMS growth medium and NMM maintenance medium. To avoid caramelization of glucose by salts, media was prepared as two separately-autoclaved solutions, one of 3:2 salts and another of 3:1 glucose, which were later combined to make the standard strength media. This procedure was followed for both the GMS growth medium and the NMM maintenance medium (Crusberg, et al., 1991).
Samples were analyzed by capillary ion analysis according the method described in this article. Results were analyzed with the Millennium software and calibration curves were generated. The original and new methods are compared below:
Waters Method N-605 for Detection of Cations in Water
|
Injection Time: |
30 seconds |
|
Injection Method: |
Hydrostatic at 9.8 cm |
|
Run Voltage: |
20 kV |
|
Capillary: |
75 mm x 60 cm uncoated fused-silica |
|
Detection: |
185 nm indirect UV |
|
Electrolyte: |
5 mM 4-methylbenzylamine, 6.5 mM a-hydroxyisobutyric acid (HIBA), natural pH (4.4) |
New Method for Detection of Transition Metal Cations in Complex Media
|
Injection Time: |
5 seconds, preceded by 30-second purge with 0.1N NaOH |
|
Injection Method: |
Hydrostatic at 9.8 cm |
|
Run Voltage: |
17.5 kV |
|
Capillary: |
75 mm x 60 cm uncoated fused-silica |
|
Detection: |
214 nm indirect UV |
|
Electrolyte: |
5 mM 4-methylbenzylamine, 10 mM a-hydroxyisobutyric acid (HIBA), natural pH (4.0) |
Results
There were some problems with using method N-605 for samples in biological media: the media in the capillary overheated resulting in current imbalance errors and system shutdown; the common alkali and alkaline earth metals masked the transition metal ion peaks; migration times were subject to "creep" with subsequent injections; and the window for appearance of the transition metals was too small. These issues were addressed as described in the sections below.
Changing the Detector Wavelength
Waters method N-605 used indirect detection at 185 nm; however, the zinc lamp we used at 214 nm resulted in better peak response for copper and zinc. Table 1 contains the areas of the copper peaks under detection at both 185 nm and 214 nm. Integration was performed by the Millennium data system with the same Integration Method on individual baseline-resolved peaks.
Copper Peak Areas (in mV*Sec) with Indirect UV Detection at 185 nm and 214 nm.
|
Concentration |
Peak Area at 185 nm |
Peak Area at 214 nm |
|
20 mg/L |
5324 |
9949 |
|
100 mg/L |
34217 |
77280 |
|
400 mg/L |
172472 |
314831 |
All areas taken from single baseline-resolved peaks.
Changing Electrolyte Composition
The electrolyte is made up of a UV-absorbing background carrier electrolyte (BCE) and a complexing agent that helps to separate the metals. The BCE used was 5 mM 4-methylbenzylamine, the same as in Waters Method N-605. The complexing agent used was 10 mM a-hydroxyisobutyric acid (HIBA) at its natural pH of 4.0. This was different from the 6.5 mM HIBA used in the Waters method. Increasing the concentration of complexing agent increased the migration time of the metals, causing them to come out later and thus resolve from the alkali and alkaline earth metals. The effect of complexing agent concentration in discussed in Chen and Cassidy, 1993; Lee and Lin, 1994; and Shi and Fritz, 1994.
Changing Injection Time
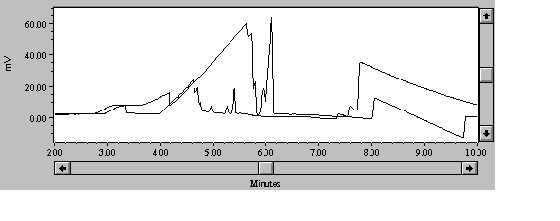
The 30-second injection time used by the Waters Method N-601 was unsuitable for the richer biological media; the alkali and alkaline earth metal cations swamped the entire detection window. Addition of methanol, lactic acid, and crown ether have been used to resolve mono- and divalent metals in the presence of 1000ppm Na+ (Shi and Fritz, 1994), but simply reducing the injection time to 5 seconds brought the Na+ peak down to a level that no longer swamped the slower-migrating metals. There was some concern that a short injection time would be less reliable, but other researchers have shown 5-second injection to be reproducible (Lee and Lin, 1994; Quang and Khaledi, 1994) and others have used 2-second (Timerbaev, et al., 1994) and 1-second injections (Chen and Cassidy, 1993). Shorter injection times result in smaller samples, and so necessarily raise detection limits; for our research, the 5-second injection was a good compromise between detection limits and sodium overload from the medium (Figure 1). Note that none of the peaks in the 30-second injection are baseline-resolved, and the "window" for the transition metal peaks goes from 1.9 minutes with the 30-second injection to 3.2 minutes with the 5-second injection.
Changing Run Voltage
Samples containing the complex media tended to run hot at 20 kV, sometimes leading to boiling within the capillary and eventual current imbalance errors which aborted the analysis. Reducing the voltage to 17.5 kV prevented the current imbalance errors while still permitting the analysis to be finished in 10 minutes. 20 kV run voltages were faster overall and were not overly noisy, but overnight sample sets were too often shut down by current imbalances. At 17.5 kV run voltage, this did not occur.
Introducing the NaOH Rinse
During use, serial injections of the high-salt media resulted in progressive retardation of migration time (Figure 2a). This may have been caused by the high concentrations of Na+ and other cations in the mixture and their propensity to coat the negatively-charged capillary wall. Timerbaev, et al., (1994) mention rinsing the capillary with 0.01N NaOH between injections, and Lee and Lin (1994) used 0.1M NaOH. We used a 30-second rinse with 0.1N NaOH between every sample injection; this dramatically improved reproducibility of migration time (Figure 2b).
Calibration Curves
Calibration curves for Cu and Zn were generated, and are shown in Figures 3 and 4. A linear curve fit was excellent, with R= 0.999, R2 = 0.998, and standard error = 1.26 x104 for copper; and R= 0.997, R2 = 0.995, and standard error = 1.48 x105 for Zn.
Other Metals
Peaks have been obtained with Cd, Co, Cr, Cu, Hg, Ni, and Zn. Detector responses vary with the metal, so detection limits vary also, but tend to be in the low (1 - 20) ppm range.
Discussion and Conclusions
Simplicity was the major concern with the development of this method. The goal was a "turn-key" method that could be used by undergraduates and grad students to run batteries of analyses reproducibly.
The strength of this method is in its ability to focus the metals in a three-minute "window" separate from the alkali and alkaline earth metals, especially the ubiquitous Na+. Other samples of complex solutions of high ionic strength include water from the Berkeley Pit Superfund site in Montana (Figure 5).
References
Chen, M. and R.M. Cassidy, 1993. Separation of Metal Ions by Capillary Electrophoresis, J. Chromatogr. 640, pp. 425-431
Crusberg, T.C., P.J. Weathers, and J.A. Mayer, 1991. Comparison of Several Biotraps for Heavy Metal Removal and Recovery from Wastewaters, Proc. HMC-Northeast '91 Conf., Hazardous Materials Control Research Institute, Bettsville, MD
Quang, C. and M.G. Khaledi, 1994. Prediction and Optimization of the Separation of Metal Cations by Capillary Electrophoresis with Indirect UV Detection, J. Chromatogr. A., 659, pp. 459-466
Shi, Y. and J.S. Fritz, 1993. Separation of Metal Ions by Capillary Electrophoresis with a Complexing Electrolyte, J. Chromatogr., 640, pp. 473-479
Shi, Y. and J.S. Fritz, 1994. New Electrolyte Systems for the Determination of Metal Cations by Capillary Zone Electrophoresis, J. Chromatogr. A., 671, pp. 429-435
Timerbaev, A.R., O.P. Semenova, P. Jandik, and G.K. Bonn, 1994. Metal Ion Capillary Electrophoresis with Direct UV Detection Effect of a Charged Surfactant on the Migration Behavior of Metal Chelates, J. Chromatogr. A., 671, pp. 419-427
Lee, Y.-H. and T.-I. Lin, 1994. Determination of Metal Cations by Capillary Electrophoresis Effect of Background Carrier and Complexing Agents, J. Chromatogr. A., 675, pp. 227-236

Figure 1. Five transition metals in GMS at 5-second and 30-second hydrostatic injection.
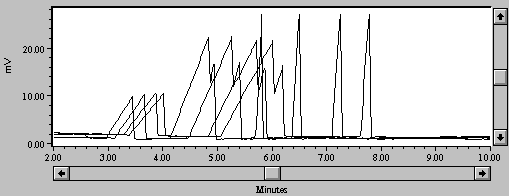
Figure 2a. Four samples of 50 mg/L Zn without 30-second rinse with 0.1N NaOH.
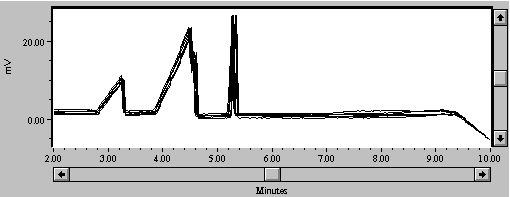
Figure 2b. Eight samples of 50 mg/L Zn with 30-second rinse with 0.1N NaOH between samples.
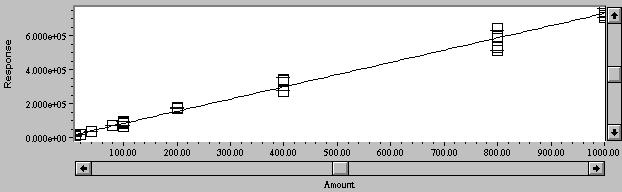
Figure 3. Calibration curve for copper in NMM medium from 10 to 1000 mg/L.
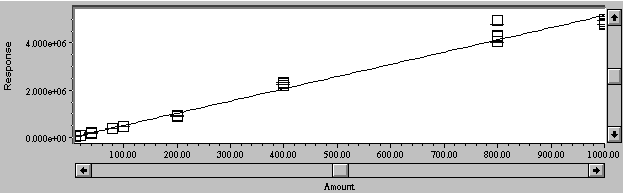
Figure 4. Calibration curve for zinc in NMM medium from 10 to 1000 mg/L.
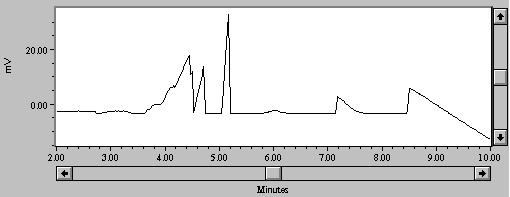
Figure 5.